
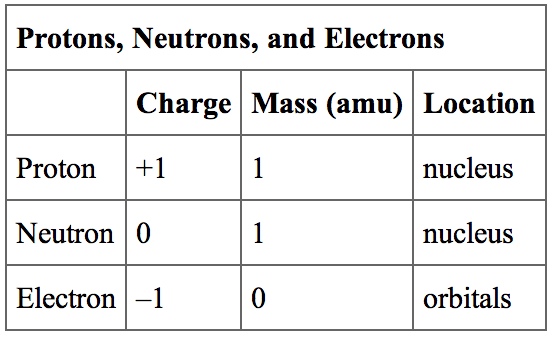
The diameter of an atom is on the order of 10 −10 m, whereas the diameter of the nucleus is roughly 10 −15 m-about 100,000 times smaller. The nucleus contains the majority of an atom’s mass because protons and neutrons are much heavier than electrons, whereas electrons occupy almost all of an atom’s volume. It was learned that an atom contains a very small nucleus composed of positively charged protons and uncharged neutrons, surrounded by a much larger volume of space containing negatively charged electrons. The development of modern atomic theory revealed much about the inner structure of atoms. Calculate average atomic mass and isotopic abundance.Define the atomic mass unit and average atomic mass.Write and interpret symbols that depict the atomic number, mass number, and charge of an atom or ion.Oligomycin inhibits ATP synthase.By the end of this section, you will be able to: Rotenone inhibits complex I, carboxin inhibits complex II, antimycin A inhibits complex III, and cyanide and CO inhibit complex IV. Ĭertain poisons can inhibit cellular oxidative phosphorylation such as rotenone, carboxin, antimycin A, cyanide, carbon monoxide (CO), sodium azide, and oligomycin. These brown fat mitochondria contain more thermogenin than other cells, allowing for increased inner mitochondrial membrane disruption and proton leakage. shivering) and are therefore at risk for hypothermia. This difference supports that brown fat is classically abundantly present in hibernating animals or newborns, who have delayed neurologic thermoregulation (ex. Brown adipose tissue has many small lipid droplets and a high concentration of mitochondria (which provide the "brown" color), in contrast to white adipose tissue, which has a single droplet. Thermogenin, also known as uncoupling protein 1 (UCP1), is found in brown adipose tissue. In the light-independent reactions, sugar is made from the ATP and NADPH from the previous reactions.

The proton gradient used to make the ATP forms via an electron transport chain. In the light-dependent reactions, light energy and water are used to make ATP, NADPH, and oxygen (O2). Photosynthesis is a metabolic process that converts light energy into chemical energy to build sugars. The energy released forms a proton gradient, which is used in chemiosmosis to make a large amount of ATP by the protein ATP-synthase.
#Charge of mole to charge of electron series#
The ETC is a collection of proteins bound to the inner mitochondrial membrane and organic molecules, which electrons pass through in a series of redox reactions, and release energy. Oxidative phosphorylation has two parts: the electron transport chain (ETC) and chemiosmosis. In the final step, the three NADH and one FADH2 amassed from the previous steps are used in oxidative phosphorylation, to make water and ATP.

The acetyl CoA is then used in the citric acid cycle, which is a chain of chemical reactions that produce CO2, NADH, flavin adenine dinucleotide (FADH2), and ATP. Each pyruvate oxidizes into acetyl CoA and an additional molecule of NADH and carbon dioxide (CO2). In glycolysis, glucose metabolizes into two molecules of pyruvate, with an output of ATP and nicotinamide adenine dinucleotide (NADH). Aerobic cellular respiration is made up of three parts: glycolysis, the citric acid (Krebs) cycle, and oxidative phosphorylation.


 0 kommentar(er)
0 kommentar(er)
